

How many moles are in 4.5 g of the salt magnesium chloride MgCl2?Įxplanation: For starters, we know that a 1-M solution contains 1 mole of solute for every 1.0 L of solution.
#MGCL2 MOLAR MASS PLUS#
So we need to add up 2 times 35.45 and then plus 1 Mg, which is 24.31. To find a molar mass, we have to go to our periodic table and add up the grams in the table for 1 mole. Our first step is to use the molar mass of magnesium chloride to convert from grams to moles. Why is formula mass equal to molar mass?īecause the definitions of both the mole and the atomic mass unit are based on the same reference substance, 12C, the molar mass of any substance is numerically equivalent to its atomic or formula weight in amu.Remember - that’s the number written under the element symbol and element name.ġ more row So in most cases, to find the molar mass of an element, you just need to look at its atomic mass (atomic weight) on the periodic table. The molar mass of an element is the mass in grams of one mole (6.02 x 10 23 particles) of the element. Where do you find the molar mass of an element? To go from molecules to moles, divide the numbers of molecules by 6.02 x 10 23. How do you use Avogadro’s number to calculate moles?Ĭonverting between molecules and moles is done by either multiplying by or dividing by Avogadro’s number: To go from moles to molecules, multiply the number of moles by 6.02 x 10 23. Weight is the measure of the gravitational force acting on a mass. One way to calculate mass: Mass = volume × density. You can also use this molarity calculator to find the mass concentration or molar mass. Substitute the known values to calculate the molarity: molarity = 5 / (1.2 * 36.46) = 0.114 mol/l = 0.114 M.

How do you calculate molar mass from molarity?Īs mass / volume = molarity * molar mass, then mass / (volume * molar mass) = molarity. So it stops reacting at the second chlorine. The 3rd ionisation potential is too high for this. The reason for this is that the first and second ionisation potentials for Mg are relatively low and that energy can be recovered by the lattice energy of MgCl2 and the electron affinity of Cl. Explanation: Equivalent weight of a solution is the molecular weight of the dissolved substance (solute) in grams divided by the valence of the solute.
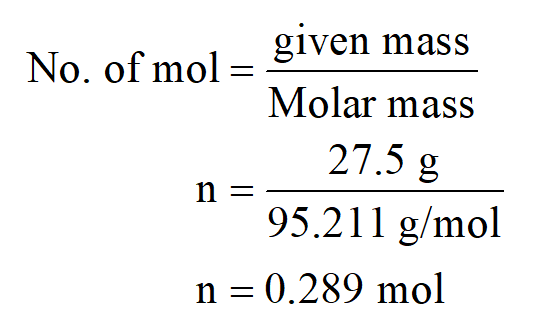
How do you find the equivalent weight of MgCl2?Īnswer: The Magnesium chloride’s equivalent weight is 47.6 gram. From here, we will get our final value that does equal do two points 10 mol. Molar mass of MgCl26H2O is 203.3027 g/mol Get control of 2022 Track your food intake, exercise, sleep and meditation for free. And we have been given the mask that is 200 grams. How many moles are there in 200 g of MgCl2?įinding number of modes is number of modes is equal to mass given divided by smaller mass, So molar mass of mg cl two is equal to 95 points 211 gram per mole.


 0 kommentar(er)
0 kommentar(er)
